My Thoughts on FSD Pharma Inc
By David Rosenberg
Executive Summary
FSD Pharma, Inc. is a publicly traded holding company, since May 2018. FSD BioSciences, Inc., a wholly-owned subsidiary, is a specialty biotech pharmaceutical R&D company focused on developing over time multiple applications of its lead compound, FSD201 ultra-micronized Palmitoylethanolamide (PEA).
The company has invested in research and development programs to seek out pharmaceutical compounds which can be used to treat multiple diseases that cause inflammation of body tissues or cells.
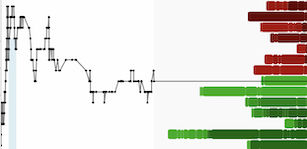
Major milestone for FSD Pharma & Shareholders was when the company was authorized by the FDA to investigate if ultra-micronized could be used to help Covid-19 patients. Covid19 has evidence of respiratory inflammation such as cytokine storm which effects tissues and delicate lung membranes. This approval by the FDA increased the company's stock (shares) demand by about 131% during that announcement investors at the time were happy the green light was given to design a phase 2a clinical trial.
The company has a strong management team and Board of Directors with extensive knowledge and experience in the development pharmaceutical drugs Dr Ed Brennan & Dr Sandra Lottes have combined work history from GSK Pharma ,Salix Pharma, Johnson & Johnson and Pfizer to name a few based on their linked in profiles.
As part of protecting its intellectual property, it had acquired the rights for ultramicroPEA and licensed it from Epitech Italy with strong patent expiring 2029-2034 and rebranded it as FSD 201. They have worldwide rights to market the drug except for Italy and Spain where Epitech will be dominant. Epitech has a phase 4 clinical trial which is currently recruiting covid19 patients in Italy,
https://clinicaltrials.gov/ct2/show/NCT04568876?term=Palmitoylethanolamide&draw=2&rank=3
FSD Pharma’s phase 2 trial in USA can be found here
https://clinicaltrials.gov/ct2/show/NCT04619706?term=Palmitoylethanolamide&draw=2&rank=8
I predict some kind of synergistic effect between the two trials that could one day expedite and lead towards commercialization but that remains to be seen and is only a theory.
Currently as of Nov 25 2020 $HUGE trading at $1.46USD with market cap of 35.8M it seems to be undervalued for a phase 2 biotech company that is dealing with inflammation
If you compare other phase 2 anti-inflammatory biotech companies that are not private most are trading above 100M MC range here are a few examples
$IVA currently trading at $434 million Market cap
$BHVN currently trading at 5.42 billion Market cap
$ATE Stock trading at 130M Market cap
$SRNE trading at $1.82 Billion Market cap
$GLPG , theravance 1.09 billion market cap
$KNSA 1.19 billion Market cap
$AMRN 1.8 Billion Market cap
FSD Pharma has a market cap of 36 M as of 11-25-2020 and looks undervalued versus other anti-inflammatory biotech companies.
Completion of financings for gross proceeds of $19.5 million USD through two registered direct offerings. As of September 30, 2020, cash & non-cash assets are $56.2 million CAD and short & long term liabilities are $13.6 million CAD.
Notably, the company shows strength and is competitive enough because it has secured enough cash and patent rights to promote its business's sustainability again this is solely my opinion.
AGP Alliance Growth Partners were the main investment bank that had raised money for FSD Pharma to advance their clinical trial efforts.
Potential investors should always ask their financial advisors on buy or sell advice however if you look at recent institutional ownership which can be found here they have been slowly accumulating some positions
WEDBUSH SECURITIES INC currently holding 64,300 shares
TWO SIGMA ADVISERS, LP currently holding 43,100 shares
SUSQUEHANNA INTERNATIONAL GROUP, LLP currently holding 10,014 shares
UBS GROUP AG currently holding 4853 shares
SIMPLEX TRADING, LLC currently holding 1747 shares
Management
FSD has a highly skilled and talented workforce comprising of 18 to 20 employees. The availability of workers with the right talent enables the company to perform well in research, innovation, and prescriptive drugs production. The company's management team also comprises a passionate team of competent managers with comprehensive industry knowledge that makes them inspire scientific innovations that lead to the discovery and development of therapeutic drugs.
The management team comprises of Raza Bokhari (CEO), Sandra Lottes (Head of Clinical Research), Zeeshan Saeed (Founder and Director), Donal Carroll (Chief Financial Officer), Sara May (Vice-President), Sandy Huard (Communications and Investor Relations), Maryann Adesso (Chief of Staff), and Edward Brennan (FSD BioSciences President). There is also a highly competent Board of Directors with the adequate breadth and the depth of expertise required to help steer its strategic vision. The board members include Gerald Goldberg (Directors), Antony Durkacz (Founder and Director), James A. Datin (Director) Hon Stephen Buyer (Director), Robert J. Ciaruffoli (Director), Larry Kaiser (Director), Zeeshan Saeed (Founder and Director), and Raza Bokhari (CEO and chairman of the Board).
This list demonstrates that the company has a rich leadership team with extensive backgrounds and a history to back up their conviction in the pharmaceutical industry.
Notably, the company has strengthened its leadership team by gathering managers and directors from different cultures, nationalities, and gender to build a world-class experience. It ensures that the company can deliver stable and positive shareholder value to its stakeholders.
Insiders also own Class B Shares which can be found here https://ceo.ca/api/sedi/?symbol=huge&amount=&transaction=&insider=
If the company succeeds one day with strong management and execution from phase 2 data points they might even be able to expand indications for different diseases.
Research Areas
FSD BioSciences holds the exclusive worldwide licensing rights (except Italy and Spain) to ultra- micronized-PEA for all conditions in all regulatory categories. It has a strong IP portfolio covering ultra-micronized composition of matter and use (2029-34 U.S. expiration).
Lead candidate is FSD201 – 600mg ultra-micronized-PEA:
• Successfully completed Phase 1 first-in-human safety and tolerability trial with no serious adverse effects discovered.
Target indications for Phase 2a trial:
• COVID-19
Potential target indication areas for Phase 2 trials:
• Osteoarthritis of the knee
• Women’s health, including endometriosis
• Chronic pain, including opioid replacement and/or sparing
The company has invested in extensive biotech pharmaceutical research and development to maximize the multiple usages of its core product FSD201 ultra-micronized palmitoylethanolamide (PEA). Ultra-micronized PEA consists of naturally occurring fatty acid amides and work on the endocannabinoid system of the human body but has nothing to do with cannabis and works in a unique way.
This compound targets the endocannabinoid system's CB2 receptors to reduce pro-inflammatory cytokines to initiate an anti-inflammatory response. FSD completed Phase 1 of the first-in-human tolerability clinical study for FSD201 that had an acceptable safety level and with no or less severe side effects. The study was considered scientific literature and approved and published in the European Union that approves the safety and tolerability of Micro-PEA. Since 2004, ultra-micro PEA is dispensed in Spain and Italy as a prescription medical food supplement.
More than 600 scientific papers attest to the physiological properties of PEA and its role as an endogenous modulator, as well as its pharmacological and therapeutic effects, specifically its anti-inflammatory profile.
PEA acts via multiple mechanisms either directly to activate PPAR-α and GPR55 or indirectly through the inhibition of FAAH, which increases endogenous levels of anandamide (AEA) and 2-arachidonoyl-glycerol (2-AG). These endocannabinoids directly activate CB2 (or CB1) receptors and TRPV1 channels (entourage effect). PEA may also activate TRPV1 channels via PPAR-α.
AEA has been shown to inhibit tumor necrosis factor-α-induced NF-kappa B activation, independent of CB1 and CB2. Saturated acylethanolamides such as PEA (an endogenous congener of AEA) may act in an analogous fashion to modify chronic inflammation in autoimmune disorders.
Nobel laureate Rita Levi-Montalcini described the importance of the activation of the inflammatory cascade and in 1993 discovered that PEA functions as a mast cell modulator by reducing mast cell migration and degranulation; thus, PEA reduces the pathological overactivation of these cells and the activity of proinflammatory cytokines (such as TNF-α and IL6), cyclooxygenase and iNOS. It is this excess immune response activity that contributes to the physiologic derangement induced by influenza viruses and sets up the pathogenesis of the "cytokine storm."
In summary, PEA down regulates hyperactive mast cells, inhibits iNOS expression and nuclear NF-kappa B translocation. It is theorized that coronavirus activates the cellular IKK/NF-kappa B signaling pathway for replication; therefore, PEA as a PPAR-α agonist may ameliorate oxidative/nitrosative stress induced by NF-kappa B and may be a suitable agent for antiviral intervention.
In addition, PEA has repeatedly been shown to down-modulate excess immune response activity that contributes to the physiologic derangement induced by viruses and help mitigate the pathogenesis of the "cytokine storm."
FSD Pharma's research and innovation prowess is unmatched. For instance, it was authorized by the Food and Drug Administration (FDA) on 1st June 2020 to submit an investigative report or New Drug Application (IND) regarding the application of FSD2O1 to treat Covid-19. Severe SARS-CoV-2 virus attack is characterized by extreme inflammation of the respiratory system, which causes cytokine storm and death. FSD was dedicated to refine and leverage the anti-inflammatory properties of FSD201 that can prevent cytokine storm that causes severe lung injury among the Covid-19 patients in critical conditions. It is believed that cannabis-derived products like CBD oil could be used in Covid-19 treatment. FSD is still striving to find another compound used along with the ultra-micronized PEA to cure Covid-19 disease.
Moreover, the company elevates its global competitiveness in the worldwide pharma industry through innovation and research dedicated to making individuals' lives better while at the same funding the company's growth. FSD BioScience strives to discover, develop, and bring to the pharma market innovative prescription drugs aligned with its micro-PEA proprietary. The company conducts several studies to find new Micro-PEA-based prescription drugs that could be used to treat multiple medical conditions. FSD BioSciences has various research areas in which it holds exclusive patent rights globally, except in Spain and Italy, to investigate micro-PEA application in the treatment of several conditions. It has a robust intellectual property (IP) portfolio protecting its ultra-micronized product between 2029 to 2034 in the US. The core target areas include osteoarthritis of the knee, opioid replacement, chronic pain, endometriosis, and women's health.
After several clinical trials and research, Ultra-Micronized PEA has been discovered to have extensive usages aligned with its analgesic and anti-inflammatory actions. Firstly, it enhances the potency and efficacy of the analgesic and anti-inflammatory therapeutic capabilities. Secondly, it can "entourage" some drugs that can significantly impact the endocannabinoid system. Thirdly, it offers clinically meaningful anti-inflammatory properly. Finally, it has substantial bioavailability and absorption clinical and preclinical efficacy and a more significant safety profile. Such properties make ultra-micronized PEA a highly marketable product because it can be used extensively to treat multiple diseases.
Extensive knowledge in FSD BioSciences has made the company excel in the development of medical cannabis. Dr. Kaiser and Dr. Bokhari have served for a long time in the pharmaceutical industry. They have accumulated the relevant resources, skills, and experiences that would make the company excel in developing cannabis prescription drugs. These and other leaders within the BioSciences department offer the company the right pool of technicians, medical researchers, innovators, and designers who conduct medical research to determine whether or not their drugs can treat specific diseases in the society. As a result, FSD can develop, monitor, coordinate, oversee, and assess internal and external clinical trials and other practices. The company has diverse competencies and capacity management in various therapeutic areas.
Conclusion
I hope that you found this collection of thoughts and research about FSD Pharma resourceful. This article offers insight of competitive advantages and capacity management, which FSD has managed to build since going public. I am happy that the company has leveraged its strengths in leadership, management, strategic partnership, patent protection rights, R&D to overcome potential challenges in the external environment. The company is doing well, and I am sure it can leverage its competitiveness to actualize its bright future. This article is not mean to give buy or sell advice and also to note I’m not a financial advisor and am not soliciting buy or sell advice. I am a FSD Pharma shareholder and tried to give a brief rundown on what the company is doing.